14 Primary Protocol
14.1 Overview
Below, we provide a straight-forward and broadly applicable protocol to achieve project goal of quantifying variability in plant-herbivore interactions. This is HerbVar’s Primary Survey Protocol. In brief, 30 randomly selected plant individuals in a site (~population) are surveyed for herbivore damage and (possibly) herbivore abundance. Data are also collected on the nearest conspecific neighbor of each plant (for a total of N = 60 plants). These methods yield estimates of variability, skew, and spatial patterns (e.g., autocorrelation) in herbivore damage. If the Primary Protocol is not feasible for a species or site, then we suggest one of the alternative protocols.
The HerbVar Primary Survey Protocol is designed to work for many common plant growth forms and contexts, so we expect most surveys to use this protocol. The primary protocol, however, will not work for every plant growth form or context, so HerbVar has multiple alternative survey protocols. These include protocols for surveying the following:
- Reproductive damage: Chapter 16
- Low density/abundance populations: Chapter 17
- Cacti and other succulents: Chapter 18
- Mature trees: Chapter 19 (surveys of immature trees (i.e., seedling/saplings) use the Primary Protocol below)
- Rhizomatous geophytes: Chapter 20
- Insect herbivores, galls, and mines: Chapter 21
If none of these alternative protocols fits the situation, then collaborators may deviate from the primary protocol. We trust collaborators to decide how to adapt the primary protocol in ways that work for their systems. We suggest, however, that collaborators strive to follow the spirit of the protocol below: randomly select at least 30 plants from a site and census them and their nearest neighbors for herbivory and herbivore data. For a dataset to be usable in the overall study, it will have to be comparable to data collected using this protocol. Collaborators who deviate from the HerbVar protocols should carefully record their methods.
14.2 Preparing to Sample
14.2.1 Select a plant species
We have developed the following objectives based on the patterns found in the first phase of data collection:
Surveys of the three focal species (Taraxacum officinale, Plantago lanceolata, and Plantago major), especially across broad environmental and/or geographic ranges
Surveys of species in the five focal families (Apocynaceae, Asteraceae, Fabaceae, Rubiaceae, and Solanaceae). We want surveys of new species within these families, especially species from new clades or with unusual growth forms. For repeat surveys of species within these families, we are prioritizing surveys from new regions, habitats, elevations, etc.
Surveys of damage to any species’ reproductive tissues (e.g., flowers, fruits, etc.).
While we welcome all surveys, data that fall under one or more of these three guidelines is particularly valuable in addressing the current scope of HerbVar’s research questions. Please refer to our more detailed HerbVar Species Selection Protocol for more information on species selection and how data contribution relates to authorship in papers utilizing those data.
14.2.1.1 A note on the ideal abundance of focal plants
The primary protocol works best for sites with at least ~90 plant individuals, which allowes for random sampling. If your site has fewer than ~90 individuals of your plant species, then please consider conducting a comprehensive census of all individuals within the site as suggested in the Low Density/Abundance Protocol. A comprehensive census, when feasible, would be even better than the protocol below. If plants are far enough apart, please take GPS coordinates for each plant. If a comprehensive census is not feasible, then please modify the primary protocol or the low-density/low-abundance guidelines to work efficiently with your species and site. Please reach out to the HerbVar coordinators if you have questions or want to check that your modifications will lead to adequate data.
14.2.2 Learn to Estimate Herbivory
First, review HerbVar’s Damage Estimation Training Guidelines (see Chapter 15), which contains valuable information on how to estimate percent damage on various leaves, the precision of estimates, and acceptable binning. Next, train and test team members with the ZAX Herbivory Trainer. This web-based application, created by Dr. Angela Moles and Zoe Xirocostas, provide a risk-free environment for testing oneself on per-leaf damage estimation. Note that the app prompts you to assess damage to the nearest percent while our protocol is slightly coarser (see Table 15.1 of the Damage Estimation Guidelines). The app has two stages - in the first you assess damage on a leaf and are immediately told how close you were to the correct amount, while in the second you are given the results after estimating herbivory on 50 leaves. Please feel free to focus on the first part of the app until you are confident (though you are of course welcome to do the second if you want extra training). Finally, download and familiarize yourself with the datasheets for this protocol. There are digital (see siteData, densityData, and plantData sheets) and printable versions to facilitate standardized data entry. If you have a question, feel free to reach out to herbvar@gmail.com.
14.2.3 Site Selection & Delineation
A “site” should be an area where a given plant species occurs at a high enough density to easily select 30 focal plants and 30 unique neighbors with our method. While new sites can be anywhere in the world, the most valuable new sites are those representing geographic regions, environmental conditions, habitats, or other ecological characteristics absent from or poorly represented in our database. This is especially important for our focal species and re-surveys of other species we already have in our database. It’s less important for new species, especially when those new species are from clades that we do not currently have in the database.
We recognize that defining the ‘edges’ of a site can be subjective and not easy. Typically, we identify an area where the focal plant species occurs at a sufficiently high density to allow for selecting 30 focal plants and 30 unique neighbors using our search methods. This is usually a relatively dense patch. Walk around and see if you see the density drop off to well below the mean density that is used to calculate radius size. This is usually quite simple, e.g., when we walk out from the center of a “site” and don’t see any individuals of the focal species within 5 m, we decide we’re at the edge of a patch. In some systems, delineating a single, sampleable population simply might not be possible (e.g., where a species covers a vast area). In these cases, collaborators should simply do their best to select a reasonable, representative area to sample.
14.2.4 Pick a time to sample
The optimal time to sample will depend on the natural history of the system. Data can be collected at any time as long as there has been some herbivory. We will use the sampling date to examine how herbivory changes seasonally (please note approximate dates for beginning and end of growing season for each survey, see siteData sheet in datasheet template). However, the most valuable surveys will be after enough time has passed for an ecologically meaningful amount of herbivory to accumulate. In strongly seasonal systems, this will be in the latter half of the growing season. But it could also be once leaves have reached maturity (e.g., for species in which most herbivory is on expanding leaves). In other systems, the best time to sample might be during or after a key life history stage (e.g., flowering). All that said, there is no perfect time to sample. Collaborators should use their knowledge to decide when to sample. Repeat sampling is acceptable.
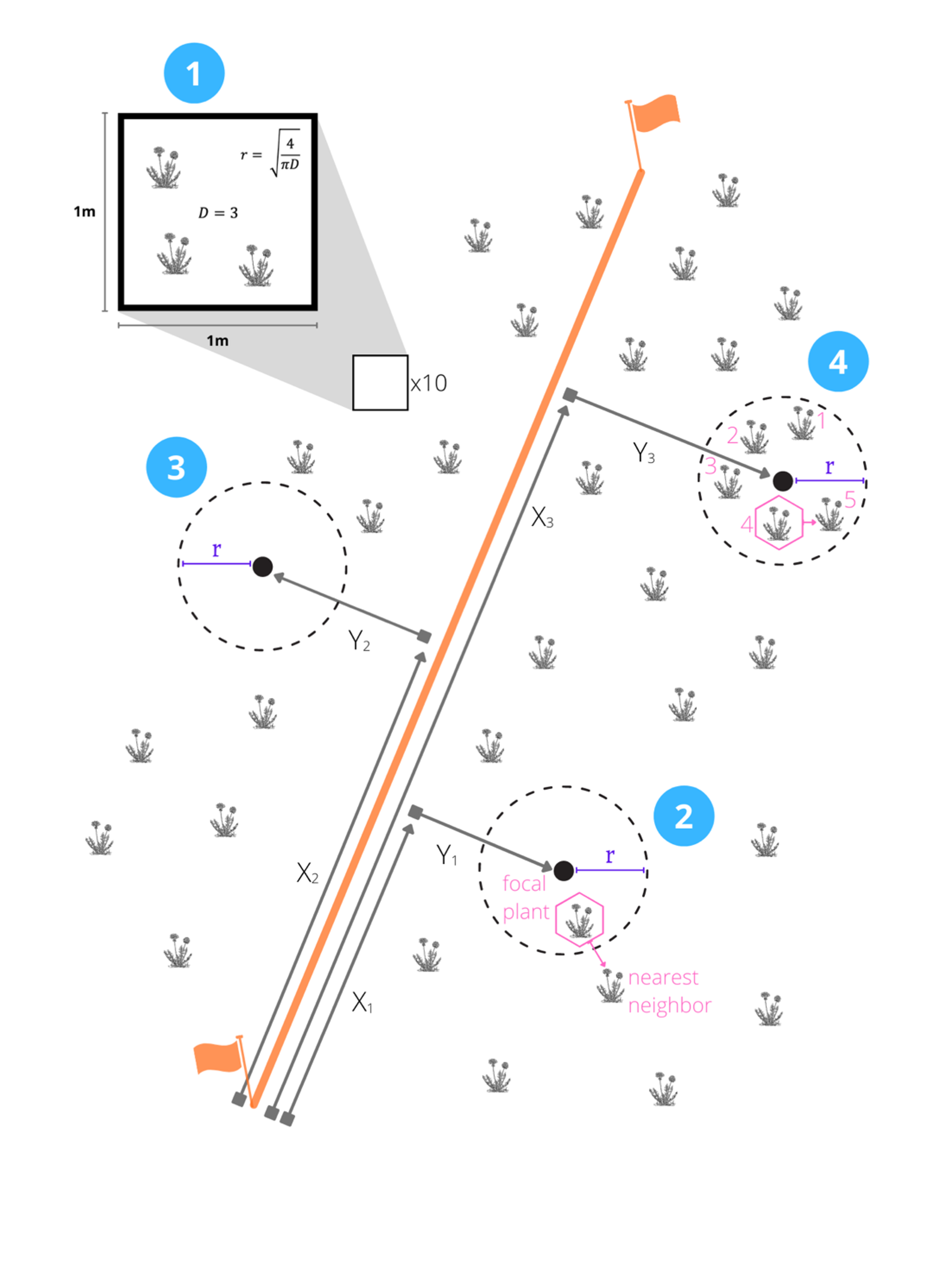
14.2.5 Determine a radius for your circular quadrats
If you are sampling one of the HerbVar focal species (Taraxacum officinale, Plantago major, Plantago lanceolata): Use a radius of 0.4 m for your quadrats. This will standardize across surveys of these same species. Note that if your populations are sparse, you may use a larger radius following the other process or pre-calculated values (Table 14.1).
If you are using any other species, use the following process to determine a quadrat radius or use the table below:
- Select 10 random locations in the site.
- At each of these points establish a 1 m
- Calculate the mean density of plants in the quadrats, D,
- Calculate a circular quadrat radius (r) that would – on average – contain 4 plants using this formula: r = 4/(πD).
This approach seeks an optimal, intermediate quadrat size that balances the costs associated with a small quadrat size (many empty quadrats) and a large quadrat size (quadrats that require counting many plant individuals).
Instead of calculating as above, you may also use this pre-calculated set of radii (Table 14.1) for non-focal species. Remember, for focal species, please use 0.4 m
14.3 Sampling
Lay a transect through the middle of the site (Figure 14.1). Record (i) the GPS coordinates of the transect origin, and (ii) the length (m), and compass direction (degrees) of transect (need to pick a coordinate system and precision)
Select center points of circular quadrats (Figure 14.1). Randomly select 40+ points in the site by selecting pairs of random numbers. One random number represents distance along the transect (0–length of transect); the other represents distance left or right of the transect (left=negative, 0=center, right=positive). These are the center points of quadrats.
Locate the center point of each quadrat, then count and record the following quadrat-level data:
The number of focal plants within r meters of the center point (see Figure 14.1). See above for explanation of how to calculate r, or use the values in Table 14.1. It may be helpful to place a stick vertically in the center of the quadrat, attach a string of r meters to the tip, and walk in a circle around the stick to help visualize the circular quadrat. Note: this includes only rooted focal plant species individuals in the quadrat.
The percent cover of focal plant species (ignore non-focal species). Note: this includes both rooted and not rooted focal plant species individuals in the quadrat but hanging over the edge from above. This percentage could be > 100% if plants overlap.
The percent cover of all non-focal plant species (ignore focal species). This percentage could be > 100% if plants overlap. If surveying understory plants, ignore forest canopy when estimating percent cover.
If the quadrat has > 0 of the focal plant species: Randomly choose 1 of the plants in the quadrat to survey (a faster alternative would be to choose the plant closest to the quadrat center, but this is recommended only if you think it will produce an unbiased sample of plants from your site. If you chose this approach, be careful about over-representing isolated plants or larger ones, which in crowded patches will be closer to more points relative to small plants). For the selected plant record:
The plant’s life-history stage (i.e., seedling, vegetative, flowering, fruiting). Note that if multiple stages are present, record all relevant stages (i.e., a plant can be both flowering and fruiting).
The size of the plant. Use judgment to pick best measure for your species (e.g., plant height from the ground to tallest living part, stem length, foliage diameter, stem diameter).
The following three measures of herbivore damage (see also Chapter 15). Note that “herbivore damage” includes damage caused by both vertebrate and invertebrate herbivores.
Presence/absence of leaf damage: Record both the total number of leaves on the plant and the number of leaves with any visible damage (> 0% herbivory). Note that we are no longer including undamaged leaves in step ii below, so the presence/absence data are vital in understanding the proportion of the plant that is damaged by herbivores. If the plant has
Estimated percent damage on 10 randomly chosen leaves with herbivore damage (> 0% herbivory). One estimate per leaf (for a total of 10 estimates). Strive to ensure the selected leaves are representative of all leaves on the plant (e.g., sample young and old leaves in proportion to frequency on plant). If desired, you may use an application to estimate damage (e.g., LeafByte, etc.). However, please make a note of that in the appropriate part of the siteData tab of the template datasheet. Note that all selected leaves should have > 0% damage (this is a change from the Phase 1 protocol). Note also that measuring only damaged leaves makes the data collected in step 1 (see above) vital in understanding per-plant damage variation.
If damage is estimated visually, and leaves are visibly damaged but damage is
Estimated percent damage across the whole plant. For example, if a plant has 4 equally sized leaves of which 2 are 50% eaten, then herbivory for the whole plant is 25% (if leaves are differnt sizes, correct this estimate by taking leaf size into account). If this measure is not feasible to collect, measure 30 leaves (instead of 10 as in step 2 above) and leave this blank. The 30 can then be used to calculate this value post hoc. Optional but valuable: separating percent damage by herbivore type or species, if possible (e.g., sucking damage versus chewing damage); add columns as needed.
Optional but encouraged: Estimate and report damage to reproductive parts (flowers/fruits/seeds) using the Protocol in Chapter 16.
Record the presence of plant diseases (i.e., pathogens). Please also estimate your confidence in your pathogen estimate and include it as a note in the provided column in the datasheet. In Phase 1, several collaborators pointed out that the difference between pathogen pressure and nutrient deficiency can be slim so this confidence estimate will be helpful in accounting for the difficulty in pathogen estimation.
Record number of leaf mines and galls per plant. If there is reason to believe that galls or mines have accumulated through multiple years (e.g., stem galls on woody perennials), please note this. If there are too many mines or galls to count individually, estimate the number per plant by tallying the number per module (e.g., stem, branch) and multiplying by number of modules. If serpentine/linear mines cannot be confidently recorded, instead count only blotch mines to record a consistent mine abundance (see visual guide at bottom of Herbivore Sampling Protocol for definitions of “serpentine” versus “blotch” mines). Optional: abundance of other externally-feeding herbivores (standardized approach; see Herbivore Sampling Protocol to decide if/how to collect these data).
Record the distance to nearest conspecific neighbor (where the nearest neighbor is the plant with the closest above ground tissue to any aboveground tissue on the focal plant).
Identify the nearest conspecific neighbor of the selected plant and record the same data as for the focal plant except for the data on “neighbor” plants (i.e., record nothing about the “neighbor’s neighbor”).
Continue visiting the randomly selected points until
14.4 After the Field
Enter your field-collected data into the “Template Excel file” (link at Section 23.1). Refer to the data Dictionary sheet if column meanings are unclear.
Use the Data Submission Portal to upload your data. The portal has numbered steps to assist the upload process (for instructions see Chapter 10).
After uploading via the submission portal, check the Completed Surveys file to ensure that your data were uploaded successfully. Uploaded data will have your entries in the sidebar of the app as the bottom-most row of that file.
14.5 Methods Notes
Modifications of this protocol may be necessary to adapt it to different systems (see “Alternate Protocols” box above). If the primary protocol won’t work for your system, please first consult our alternative protocols. If our alternative protocols do not solve the issues, then you may adapt the primary protocol as needed. Whatever you do, please record methods carefully and strive to follow the spirit of the protocol and produce comparable data.
Collaborators have reported that one survey (~60 plants) takes between 0.5 and 2 person-days (i.e., 4-16 hours) using the methods above (after a species and site have already been selected).
We select 40 quadrat center points (instead of 30) so that we have extra points ready in case some quadrats are empty. If you predict that many quadrats will be empty (e.g., in a very spatially clumped population of plants), then select more points (e.g., 60 points). (Remember the goal is to have 30 focal plants sampled, plus their nearest neighbors).
Sometimes, especially in small populations, a focal plant ends up being another focal plant’s neighbor. This is fine. Just note and keep going. If you have time, you can add an extra focal plant at the end (but this isn’t totally necessary).
For clonal plants, we have been calling stems “individuals” if they are not connected aboveground. When looking for above ground connections, we clear away detritus, but we do not dig or move soil. There is also an alternative protocol for surveying such species (see Chapter 20)
Please see our Damage Estimation Training Guidelines for guidelines on how to estimate herbivore damage. Here are two tips:
Sometimes discerning herbivore damage from physical damage (e.g., wind, trampling) is tricky. We do the best we can. We look at things like how jagged the cut edges are and if they travel past the missing area into the remaining leaf tissue (which would suggest the damage may have been physical).
Another challenge is old damage that occurred when leaves were still expanding. This could potentially make the area removed seem larger than it was. If we suspect something like this happened, then we try to bend the leaf back into shape to see if it seems like the missing area expanded over time.
We will accept surveys that only assess damage and do not identify herbivores. This will allow people without insect ID skills to participate in the study.
14.6 Common Garden Data
Common gardens are a powerful tool for studying plant–herbivore interactions. Several collaborators have proposed including them in HerbVar, and we would like to try if we can get enough data. To be applicable to this study a common garden’s design would have to be random with respect to genotype. If a garden was somehow stratified with blocks containing repeated instances of, e.g., different levels of leaf toughness, then damage distributions will not be comparable to damage from wild populations. We may still be able to use such datasets, but only if we have enough to use them in a separate analysis. Please get in touch if you would like to contribute common garden data.